Module 19: Metabolism and Nutrition
Lesson 1: Overview of Metabolic Reactions
Tổng Quan Về Phản Ứng Chuyển Hóa
Medical Terminology: Metabolism and Nutrition
absorptive state
acetyl coenzyme A (acetyl CoA)
anabolic hormones
anabolic reactions
ATP synthase
basal metabolic rate (BMR)
beta (β)-hydroxybutyrate
beta (β)-oxidation
bile salts
biosynthesis reactions
body mass index (BMI)
calorie
catabolic hormones
catabolic reactions
cellular respiration
cholecystokinin (CCK)
chylomicrons
chymotrypsin
chymotrypsinogen
citric acid cycle
conduction
convection
elastase
electron transport chain (ETC)
energy-consuming phase
energy-yielding phase
enterokinase
evaporation
FADH2
fatty acid oxidation
flavin adenine dinucleotide (FAD)
glucokinase
gluconeogenesis
glucose-6-phosphate
glycogen
glycolysis
hexokinase
hydroxymethylglutaryl CoA (HMG CoA)
inactive proenzymes
insulin
ketone bodies
Krebs cycle
lipogenesis
lipolysis
metabolic rate
metabolism
minerals
monoglyceride molecules
monosaccharide
NADH
nicotinamide adenine dinucleotide (NAD)
oxidation
oxidation-reduction reaction
oxidative phosphorylation
pancreatic lipases
pepsin
polysaccharides
postabsorptive state
proteolysis
pyruvate
radiation
reduction
salivary amylase
secretin
sodium bicarbonate
terminal electron acceptor
thermoneutral
thermoregulation
transamination
tricarboxylic acid cycle (TCA)
triglycerides
trypsin
trypsinogen
urea cycle
vitamins
OpenStax. (2022). Anatomy and Physiology 2e. Rice University. Retrieved June 15, 2023. ISBN-13: 978-1-711494-06-7 (Hardcover) ISBN-13: 978-1-711494-05-0 (Paperback) ISBN-13: 978-1-951693-42-8 (Digital). License: Attribution 4.0 International (CC BY 4.0). Access for free at openstax.org.

Adenosine triphosphate (ATP) is the energy molecule of the cell. During catabolic reactions, ATP is created and energy is stored until needed during anabolic reactions.
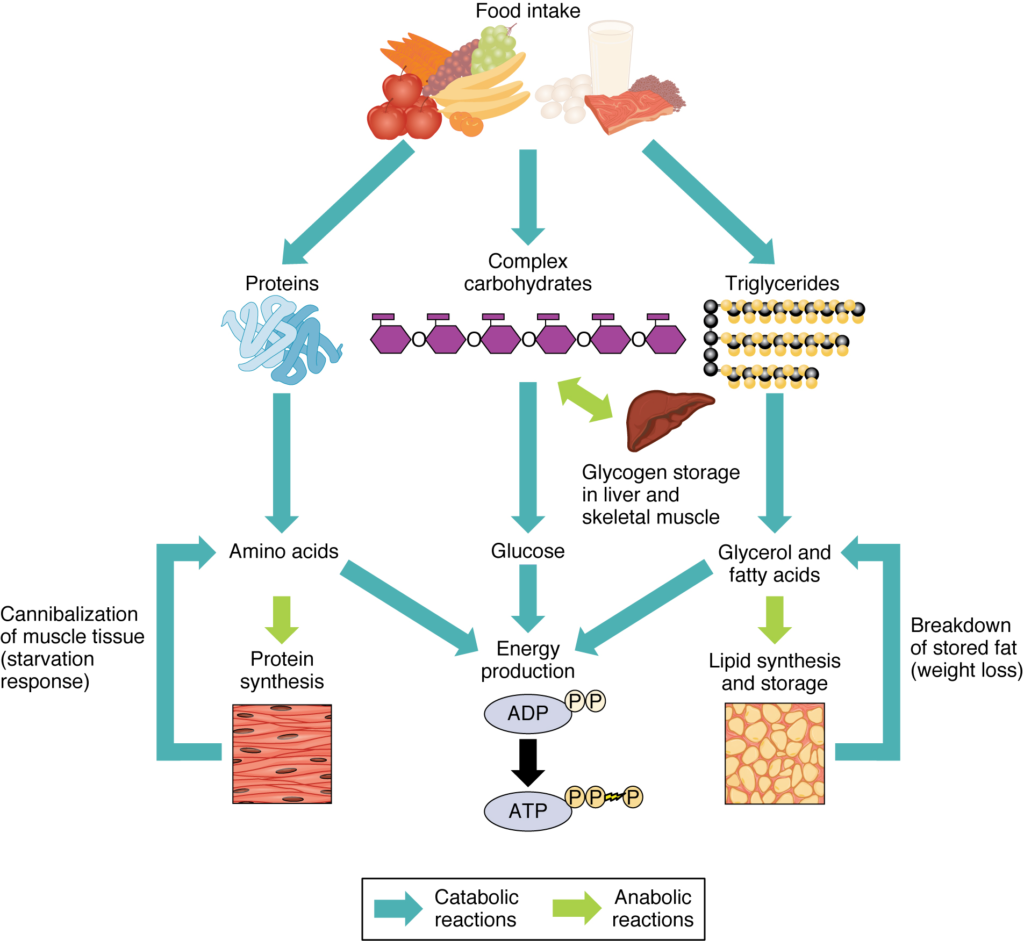
During catabolic reactions, proteins are broken down into amino acids, lipids are broken down into fatty acids, and polysaccharides are broken down into monosaccharides. These building blocks are then used for the synthesis of molecules in anabolic reactions.
| Hormone | Function |
|---|---|
| Cortisol | Released from the adrenal gland in response to stress; its main role is to increase blood glucose levels by gluconeogenesis (breaking down fats and proteins) |
| Glucagon | Released from alpha cells in the pancreas either when starving or when the body needs to generate additional energy; it stimulates the breakdown of glycogen in the liver to increase blood glucose levels; its effect is the opposite of insulin; glucagon and insulin are a part of a negative-feedback system that stabilizes blood glucose levels |
| Adrenaline/epinephrine | Released in response to the activation of the sympathetic nervous system; increases heart rate and heart contractility, constricts blood vessels, is a bronchodilator that opens (dilates) the bronchi of the lungs to increase air volume in the lungs, and stimulates gluconeogenesis |
| Hormone | Function |
|---|---|
| Growth hormone (GH) | Synthesized and released from the pituitary gland; stimulates the growth of cells, tissues, and bones |
| Insulin-like growth factor (IGF) | Stimulates the growth of muscle and bone while also inhibiting cell death (apoptosis) |
| Insulin | Produced by the beta cells of the pancreas; plays an essential role in carbohydrate and fat metabolism, controls blood glucose levels, and promotes the uptake of glucose into body cells; causes cells in muscle, adipose tissue, and liver to take up glucose from the blood and store it in the liver and muscle as glycogen; its effect is the opposite of glucagon; glucagon and insulin are a part of a negative-feedback system that stabilizes blood glucose levels |
| Testosterone | Produced by the testes in males and the ovaries in females; stimulates an increase in muscle mass and strength as well as the growth and strengthening of bone |
| Estrogen | Produced primarily by the ovaries, it is also produced by the liver and adrenal glands; its anabolic functions include increasing metabolism and fat deposition |
Script:
- In the body, metabolism is the sum of all catabolic reactions, meaning break down processes, and anabolic reactions, meaning synthesis processes.
- The metabolic rate measures the amount of energy used to maintain life.
- An organism must ingest a sufficient amount of food to maintain its metabolic rate if the organism is to stay alive for very long.
- Catabolic reactions break down larger molecules, such as carbohydrates, lipids, and proteins from ingested food, into their constituent smaller parts.
- They also include the breakdown of ATP, which releases the energy needed for metabolic processes in all cells throughout the body.
- Anabolic reactions, or biosynthetic reactions, synthesize larger molecules from smaller constituent parts, using ATP as the energy source for these reactions.
- Anabolic reactions build bone, muscle mass, and new proteins, fats, and nucleic acids.
- Oxidation-reduction reactions transfer electrons across molecules by oxidizing one molecule and reducing another, and collecting the released energy to convert phosphatidylinositol and ADP into ATP.
- Errors in metabolism alter the processing of carbohydrates, lipids, proteins, and nucleic acids, and can result in a number of disease states.
Ấn vào ô bên dưới để đánh dấu bạn đã hoàn thành bài học này
Quá dữ! Tiếp tục duy trì phong độ nhé!