Module 21: Bone Tissue and the Skeletal System
Lesson 4: Bone Formation and Development
Quá Trình Hình Thành Và Phát Triển Xương
Dưới đây là danh sách những thuật ngữ Y khoa của module Bone Tissue and the Skeletal System.
Khái quát được số lượng thuật ngữ sẽ xuất hiện trong bài đọc và nghe sẽ giúp bạn thoải mái tiêu thụ nội dung hơn. Sau khi hoàn thành nội dung đọc và nghe, bạn hãy quay lại đây và luyện tập (practice) để quen dần các thuật ngữ này. Đừng ép bản thân phải nhớ các thuật ngữ này vội vì bạn sẽ gặp và ôn lại danh sách này trong những bài học (lesson) khác của cùng một module.
Medical Terminology: Bone Tissue and the Skeletal System
articular cartilage
thin layer of cartilage covering an epiphysis; reduces friction and acts as a shock absorber
articulation
where two bone surfaces meet
bone
hard, dense connective tissue that forms the structural elements of the skeleton
canaliculi
(singular = canaliculus) channels within the bone matrix that house one of an osteocyte’s many cytoplasmic extensions that it uses to communicate and receive nutrients
cartilage
semi-rigid connective tissue found on the skeleton in areas where flexibility and smooth surfaces support movement
central canal
longitudinal channel in the center of each osteon; contains blood vessels, nerves, and lymphatic vessels; also known as the Haversian canal
closed reduction
manual manipulation of a broken bone to set it into its natural position without surgery
compact bone
dense osseous tissue that can withstand compressive forces
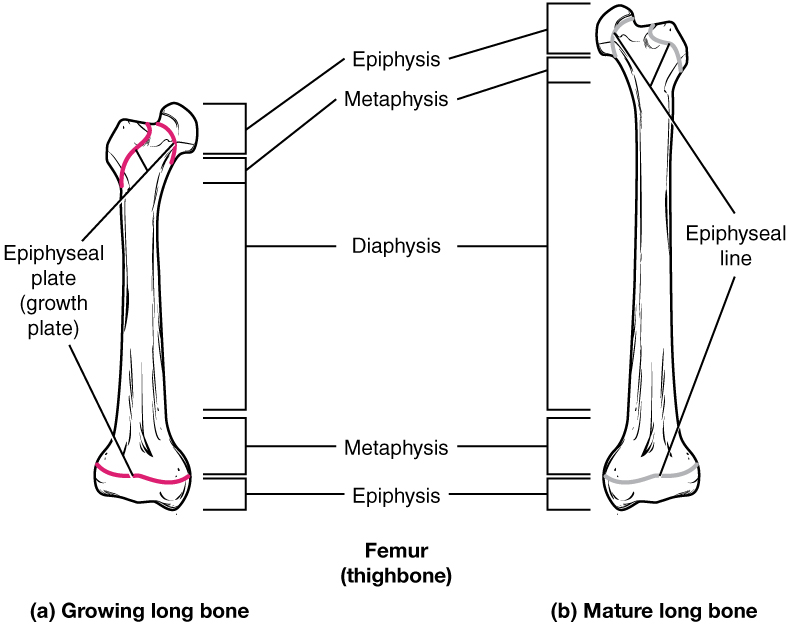
diaphysis
tubular shaft that runs between the proximal and distal ends of a long bone
diploë
layer of spongy bone, that is sandwiched between two the layers of compact bone found in flat bones
endochondral ossification
process in which bone forms by replacing hyaline cartilage
endosteum
delicate membranous lining of a bone’s medullary cavity
epiphyseal line
completely ossified remnant of the epiphyseal plate
epiphyseal plate
(also, growth plate) sheet of hyaline cartilage in the metaphysis of an immature bone; replaced by bone tissue as the organ grows in length
epiphysis
wide section at each end of a long bone; filled with spongy bone and red marrow
external callus
collar of hyaline cartilage and bone that forms around the outside of a fracture
flat bone
thin and curved bone; serves as a point of attachment for muscles and protects internal organs
fracture
broken bone
fracture hematoma
blood clot that forms at the site of a broken bone
hematopoiesis
production of blood cells, which occurs in the red marrow of the bones
hole
opening or depression in a bone
hypercalcemia
condition characterized by abnormally high levels of calcium
hypocalcemia
condition characterized by abnormally low levels of calcium
internal callus
fibrocartilaginous matrix, in the endosteal region, between the two ends of a broken bone
intramembranous ossification
process by which bone forms directly from mesenchymal tissue
irregular bone
bone of complex shape; protects internal organs from compressive forces
lacunae
(singular = lacuna) spaces in a bone that house an osteocyte
long bone
cylinder-shaped bone that is longer than it is wide; functions as a lever
medullary cavity
hollow region of the diaphysis; filled with yellow marrow
modeling
process, during bone growth, by which bone is resorbed on one surface of a bone and deposited on another
nutrient foramen
small opening in the middle of the external surface of the diaphysis, through which an artery enters the bone to provide nourishment
open reduction
surgical exposure of a bone to reset a fracture
orthopedist
doctor who specializes in diagnosing and treating musculoskeletal disorders and injuries
osseous tissue
bone tissue; a hard, dense connective tissue that forms the structural elements of the skeleton
ossification
(also, osteogenesis) bone formation
ossification center
cluster of osteoblasts found in the early stages of intramembranous ossification
osteoblast
cell responsible for forming new bone
osteoclast
cell responsible for resorbing bone
osteocyte
primary cell in mature bone; responsible for maintaining the matrix
osteogenic cell
undifferentiated cell with high mitotic activity; the only bone cells that divide; they differentiate and develop into osteoblasts
osteoid
uncalcified bone matrix secreted by osteoblasts
osteon
(also, Haversian system) basic structural unit of compact bone; made of concentric layers of calcified matrix
osteoporosis
disease characterized by a decrease in bone mass; occurs when the rate of bone resorption exceeds the rate of bone formation, a common occurrence as the body ages
perforating canal
(also, Volkmann’s canal) channel that branches off from the central canal and houses vessels and nerves that extend to the periosteum and endosteum
perichondrium
membrane that covers cartilage
periosteum
fibrous membrane covering the outer surface of bone and continuous with ligaments
primary ossification center
region, deep in the periosteal collar, where bone development starts during endochondral ossification
projection
bone markings where part of the surface sticks out above the rest of the surface, where tendons and ligaments attach
proliferative zone
region of the epiphyseal plate that makes new chondrocytes to replace those that die at the diaphyseal end of the plate and contributes to longitudinal growth of the epiphyseal plate
red marrow
connective tissue in the interior cavity of a bone where hematopoiesis takes place
remodeling
process by which osteoclasts resorb old or damaged bone at the same time as and on the same surface where osteoblasts form new bone to replace that which is resorbed
reserve zone
region of the epiphyseal plate that anchors the plate to the osseous tissue of the epiphysis
secondary ossification center
region of bone development in the epiphyses
sesamoid bone
small, round bone embedded in a tendon; protects the tendon from compressive forces
short bone
cube-shaped bone that is approximately equal in length, width, and thickness; provides limited motion
skeletal system
organ system composed of bones and cartilage that provides for movement, support, and protection
spongy bone
(also, cancellous bone) trabeculated osseous tissue that supports shifts in weight distribution
trabeculae
(singular = trabecula) spikes or sections of the lattice-like matrix in spongy bone
yellow marrow
connective tissue in the interior cavity of a bone where fat is stored
zone of calcified matrix
region of the epiphyseal plate closest to the diaphyseal end; functions to connect the epiphyseal plate to the diaphysis
zone of maturation and hypertrophy
region of the epiphyseal plate where chondrocytes from the proliferative zone grow and mature and contribute to the longitudinal growth of the epiphyseal plate
Dưới đây là các bài văn nằm ở bên trái. Ở bên phải là các bài luyện tập (practice) để đánh giá khả năng đọc hiểu của bạn. Sẽ khó khăn trong thời gian đầu nếu vốn từ vựng của bạn còn hạn chế, đặc biệt là từ vựng Y khoa. Hãy kiên nhẫn và đọc nhiều nhất có kể, lượng kiến thức tích tụ dần sẽ giúp bạn đọc thoải mái hơn.
Dưới đây là video và các luyện tập (practice) của bài này. Nghe là một kĩ năng khó, đặc biệt là khi chúng ta chưa quen nội dung và chưa có nhạy cảm ngôn ngữ. Nhưng cứ đi thật chậm và đừng bỏ cuộc.
Dưới đây là phần bàn luận. Bạn có thể tự do đặt câu hỏi, bổ sung kiến thức, và chia sẻ trải nghiệm của mình.
Subscribe
Login
0 Comments
Ấn vào ô bên dưới để đánh dấu bạn đã hoàn thành bài học này
Quá dữ! Tiếp tục duy trì phong độ nhé!